ELIGARD is indicated for the palliative treatment
of advanced prostate cancer
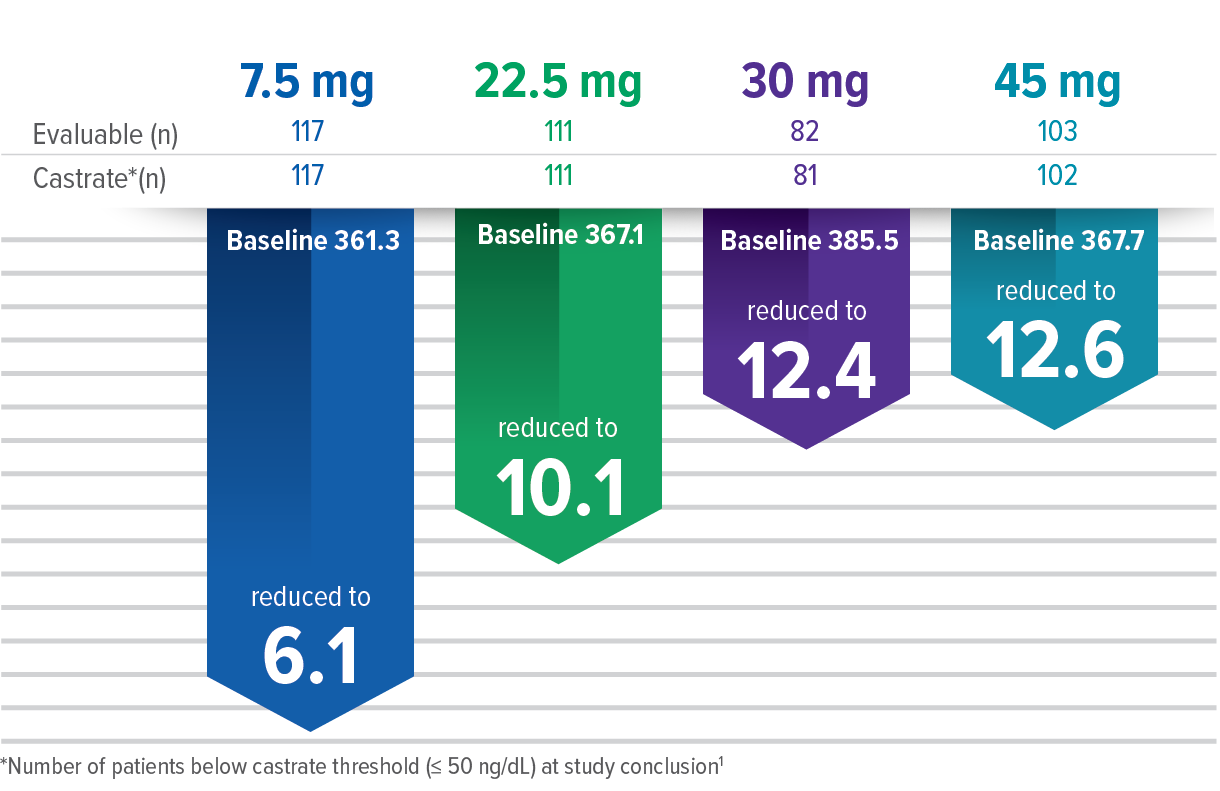
Testosterone Level (ng/dL) Achieved Across All Doses at Study Conclusion2
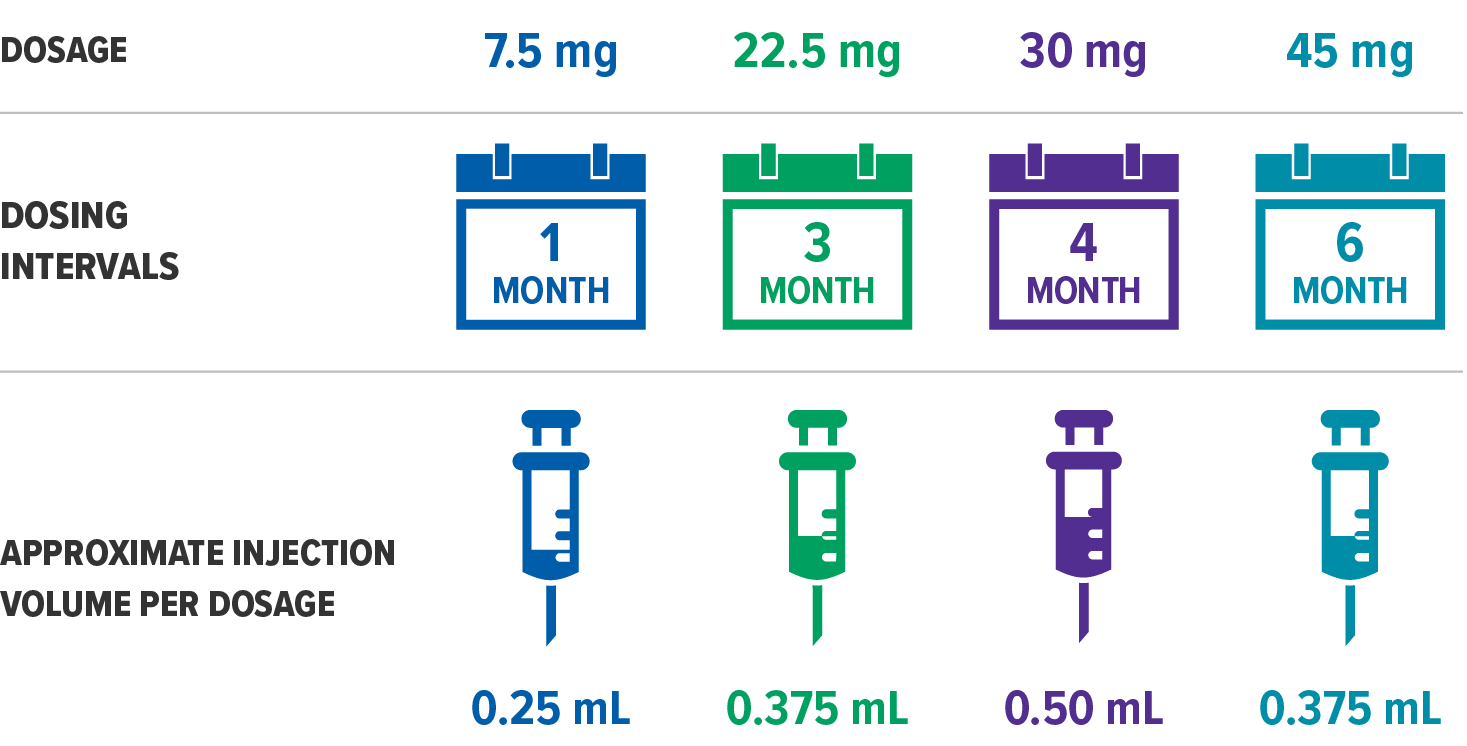
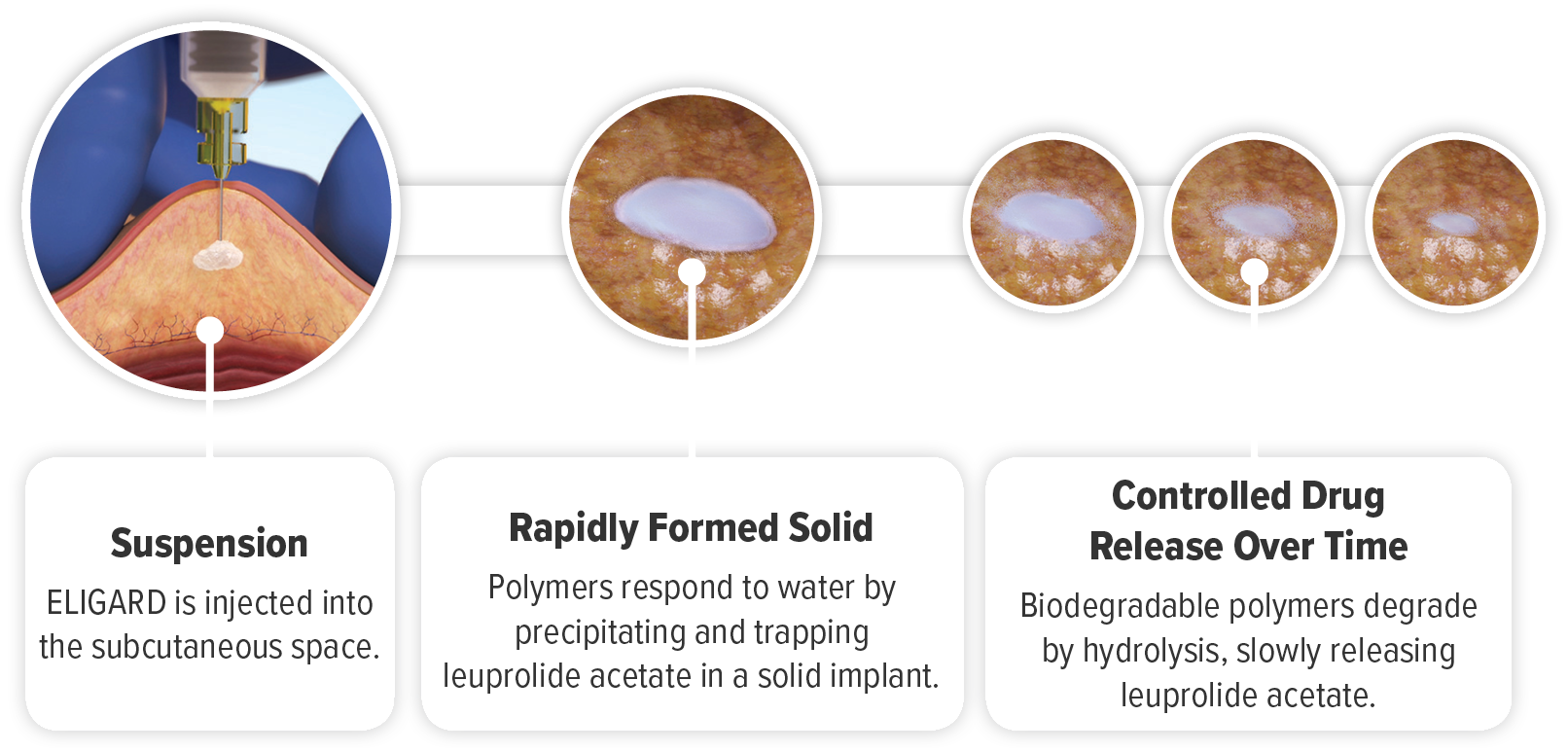
The ATRIGEL® Delivery System Providing Controlled Release1
Store at 2 – 8 °C (35.6 – 46.4 °F). Once outside the refrigerator this product may be stored in its original packaging at room temperature 15 – 30 °C (59 – 86 °F) for up to eight weeks prior to mixing and administration.
- Known hypersensitivity to GnRH, GnRH agonist analogs or any of the components of ELIGARD®. Anaphylactic reactions to synthetic GnRH or GnRH agonist analogs have been reported in the literature.
- Tumor Flare: Transient increase in serum levels of testosterone during treatment may result in worsening of symptoms or onset of new signs and symptoms during the first few weeks of treatment, including bone pain, neuropathy, hematuria, bladder outlet obstruction, ureteral obstruction, or spinal cord compression. Monitor patients at risk closely and manage as appropriate
- Laboratory Tests: Response to ELIGARD® should be monitored by periodic measurement of serum concentrations of testosterone and prostate specific antigen. In the majority of patients, testosterone levels increased above Baseline during the first week, declining thereafter to Baseline levels or below by the end of the second or third week. Castrate levels were generally reached within two to four weeks. Castrate testosterone levels were maintained for the duration of the treatment with ELIGARD® 7.5 mg. No increases to above the castrate level occurred in any of the patients. Castrate levels were generally maintained for the duration of treatment with ELIGARD® 22.5 mg
- Hyperglycemia and diabetes: Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH analogs. Monitor blood glucose level and/or glycosylated hemoglobin (HbA1c) and manage according to current clinical practice
- Cardiovascular diseases: Increased risk of myocardial infarction, sudden cardiac death and stroke has been reported in men. Monitor for cardiovascular disease and manage according to current clinical practice
- Effect on QT/QTc Interval: Androgen deprivation therapy may prolong the QT/QTc interval. Consider risks and benefits
- Embryo-Fetal Toxicity: Based on findings in animal studies and mechanism of action, leuprolide acetate may cause fetal harm when administered to a pregnant woman
- Convulsions: Have been observed in patients with or without a history of predisposing factors. Manage convulsions according to current clinical practice
