Not an actual patient.
Not an actual patient.
Unique in situ polymeric extended-release delivery system enables sustained and controlled release of leuprolide acetate as it safely biodegrades1

leuprolide acetate
PLG copolymer
N-methyl-2-pyrrolidone (NMP)
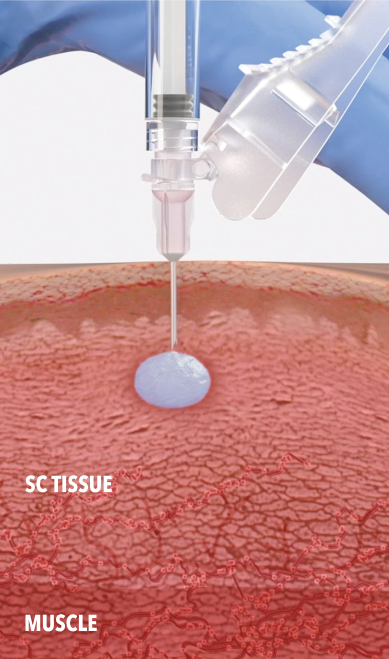
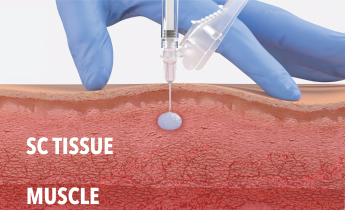
ELIGARD being injected into SC tissue and solidifying in situ.
ELIGARD in situ, slowly biodegrading over the dosing period.

Inject ELIGARD at a 90° angle into subcutaneous tissue

Virtual training sessions include a demonstration of the mixing and administration of ELIGARD. The clinical trainer will answer any questions you may have.
ELIGARD is a refrigerated product and should be stored at 2 °C to 8 °C (36-45 °F) but can be left at room temperature for up to 8 weeks (56 days) prior to mixing and administration2
If the product is NOT administered within 30 minutes of mixing, it must be discarded2