Not an actual patient.
Not an actual patient.



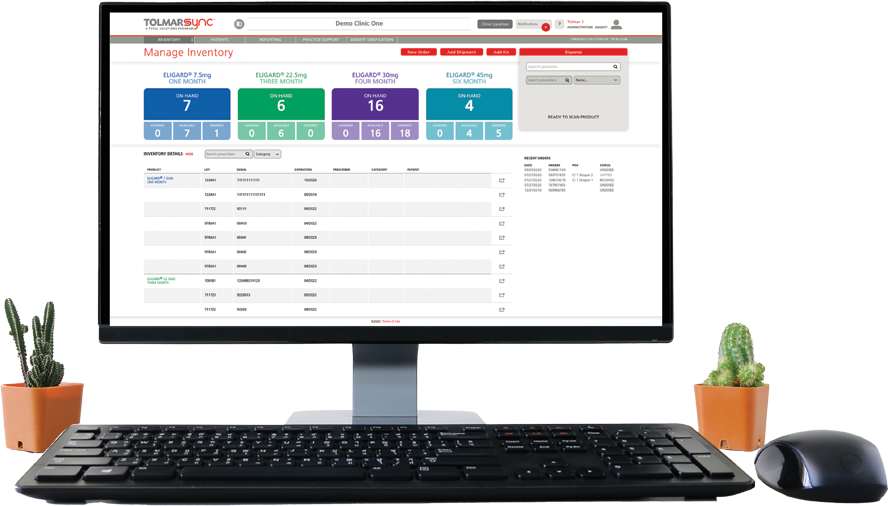
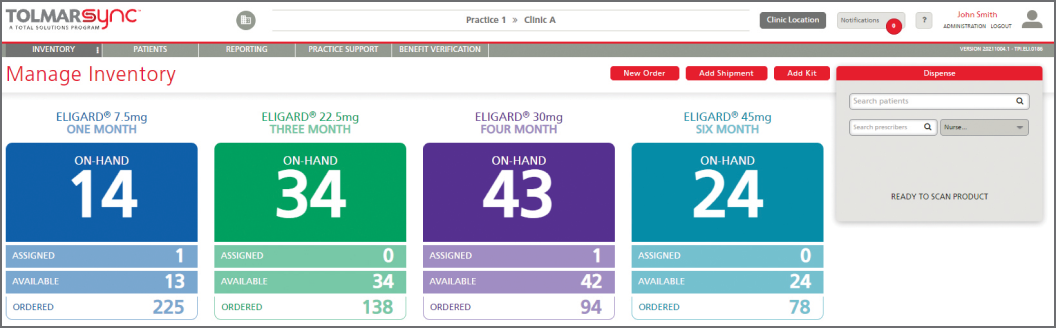
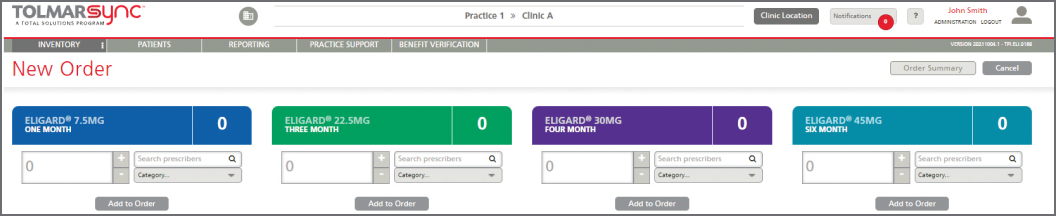
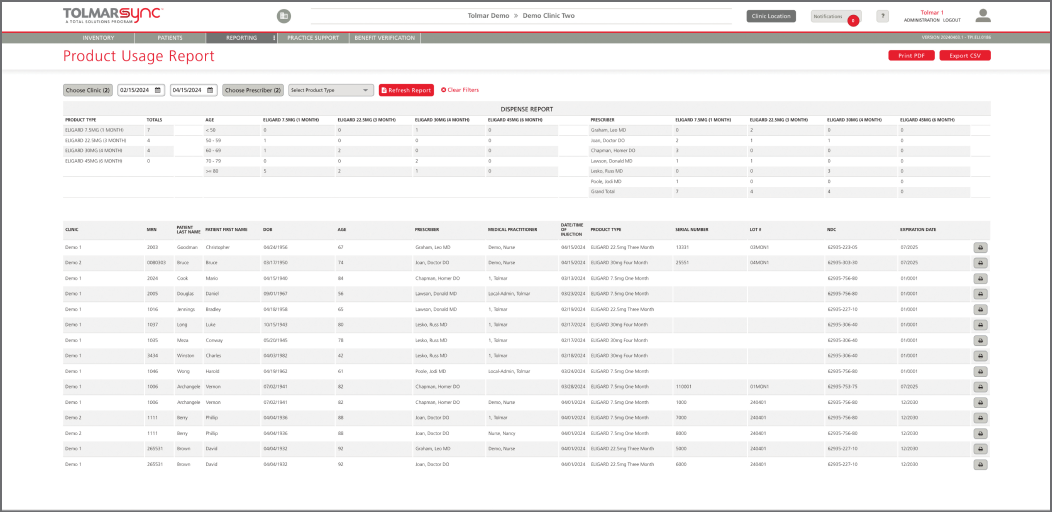
The program also provides solutions to common barriers such as injecting a patient too early, issues in billing reconciliation, and missing GnRH patients. This is done through:
Establish better prevention and management of the adverse effects of ADT through detailed step-by-step roadmaps for healthcare providers and patients, with recommendations for assessment, monitoring, counseling, and referral
Provide patients with detailed information about ADT, including a pre-treatment initiation checklist as well as guidance about what to expect and what will be assessed before and during treatment
Encourage patients with prostate cancer to implement and sustain healthy dietary and exercise practices in addition to taking other steps to enhance quality of life
This roadmap will show you how!
PROSTATE CANCER 360 IS CONTINUING TO EXPAND AND PROVIDE INFORMATION ABOUT ADT TREATMENT. LEARN MORE AT PROSTATECANCER360.COM
Tolmar is dedicated to meeting the needs of physicians, nurses, residents, and pharmacists in urology and oncology by offering peer-to-peer educational programs.

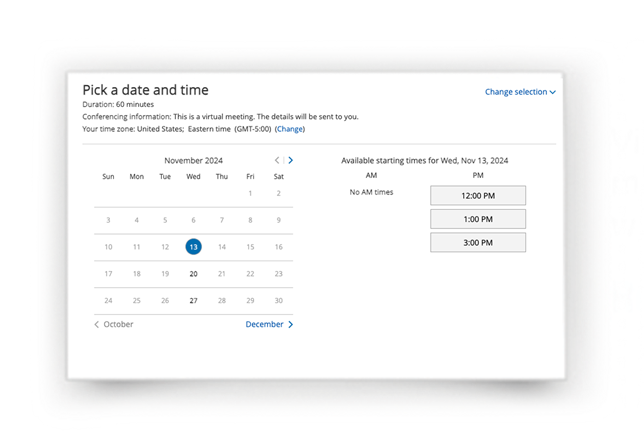
Join ELIGARD’s weekly virtual training sessions for a demonstration of the mixing and administration process. A clinical trainer will be on hand to answer any questions you may have.
Find resources below you can share with your patients as they receive treatment with ELIGARD.

Provide patients with a comprehensive resource to help them navigate their way through prostate cancer treatment.

Give this guide to your patients so they know what they can expect with ELIGARD treatment.

Share the patient roadmap from PC360 with your patients to help them prepare for ADT and learn how to navigate their diagnosis.
Lessons are completed at the pace of the user, and patients are able to download and print lessons to keep with them as they control their own progress.

The IncreMENtal Nurse Brochure helps nurses prepare their patients for the IncreMENtal program by providing information covered in the program along with sample lessons, how to get patients to adhere to habit building, and more.
American Urological Association
(AUA)
Society of Urologic Oncology
(SUO)
Veterans Prostate Cancer Awareness (VPCa)
Large Urology Group Practice Association (LUGPA)
Society of Urologic Nurses and Associates (SUNA)
American Society of Clinical Oncology (ASCO)
ZERO Prostate Cancer
We offer a full line of patient support services, including benefit verification; assistance with prior authorizations, appeals, and billing and coding inquiries; patient education materials; and our Patient Assistance Program (PAP).
ELIGARD consistently has been one of the lowest wholesale acquisition costs (WACs) in the GnRH market.
*Costs are standardized to represent one month of therapy.
TOLMAR IS COMMITTED TO KEEPING ELIGARD RESPONSIBLY PRICED
ADT, androgen deprivation therapy; GnRH, gonadotropin-releasing hormone.
Reference: 1. Data on file. Tolmar, Inc.; 2025.