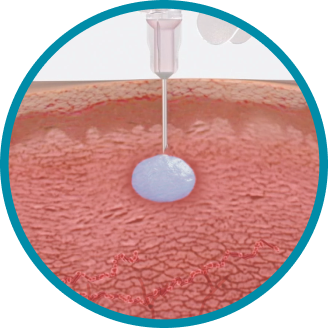
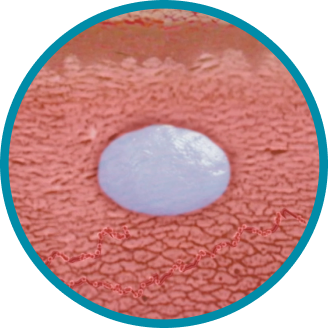
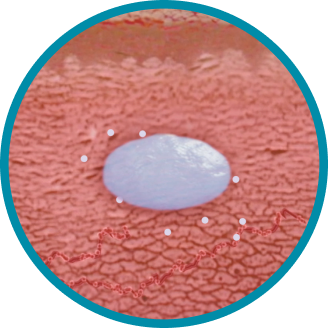
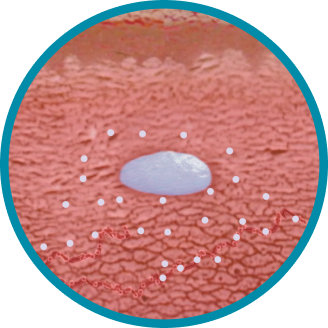
CONSISTENT, RELIABLE, AND LONG-ACTING T SUPPRESSION ENABLED BY THE PROPRIETARY DELIVERY SYSTEM OF ELIGARD®
Unique in situ polymeric extended-release delivery system enables sustained and controlled release of leuprolide acetate as it safely biodegrades1