SET YOUR SIGHTS ON FLEXIBILITY, WITH 4 DOSING OPTIONS BASED ON YOUR PATIENTS’ NEEDS
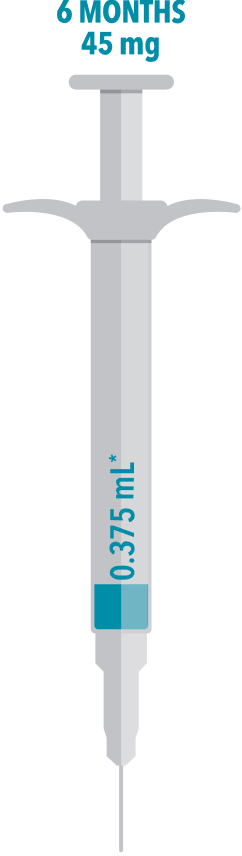
ELIGARD® comes in 1-, 3-, 4-, and 6-month doses, allowing for as few as 2 injections per year—which can align with your patients’ scheduled checkups1
Not an actual patient.
ELIGARD® comes in 1-, 3-, 4-, and 6-month doses, allowing for as few as 2 injections per year—which can align with your patients’ scheduled checkups1
The unique delivery technology of ELIGARD enables one of the smallest injection volumes available—helping provide less discomfort to your patients2



Use the Dosing Calculator tool to instantly see when your patient is due for their next dose by inputting the date of their latest treatment and prescribed dose.
References: 1. ELIGARD (leuprolide acetate). Prescribing Information. Tolmar, Inc. 2024. 2. Prettyman J, Engel L, Boldt-Houle DM, Atkinson S, Wilt W. Personalizing treatment in the delivery of care by nurses to patients with prostate cancer. Urologic Nursing. 2019;39(2):83-99.